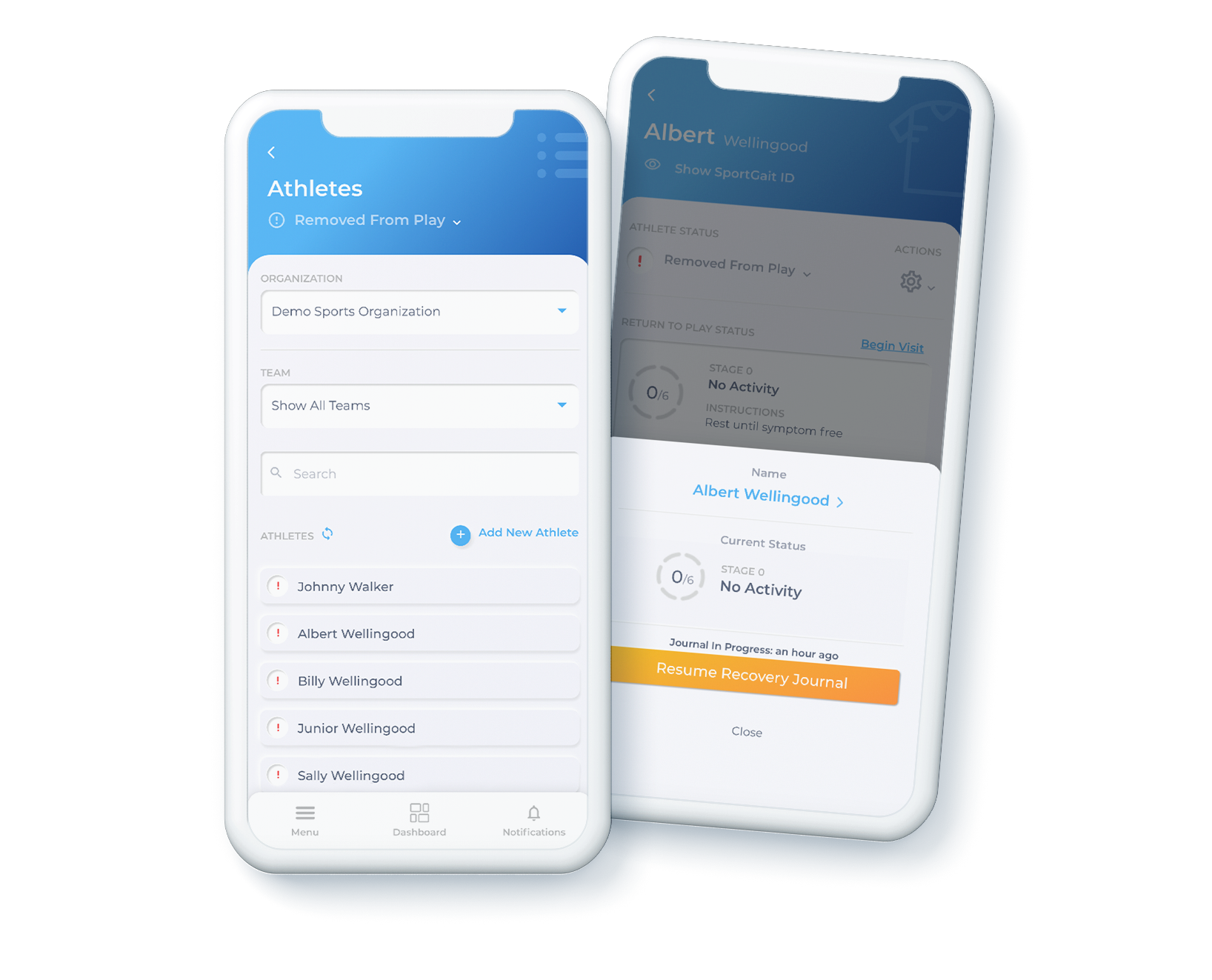
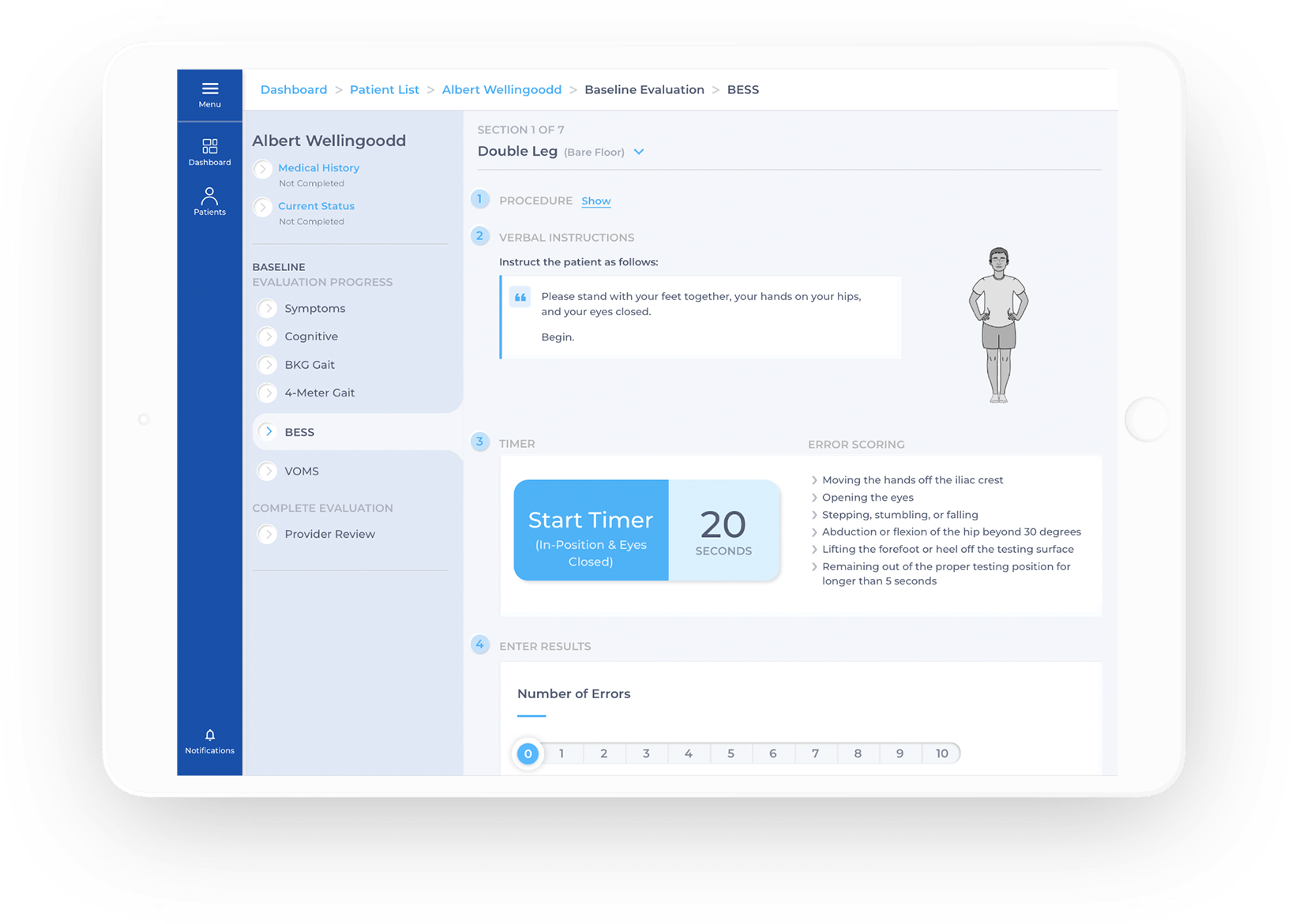
Baseline. Document. Measure.
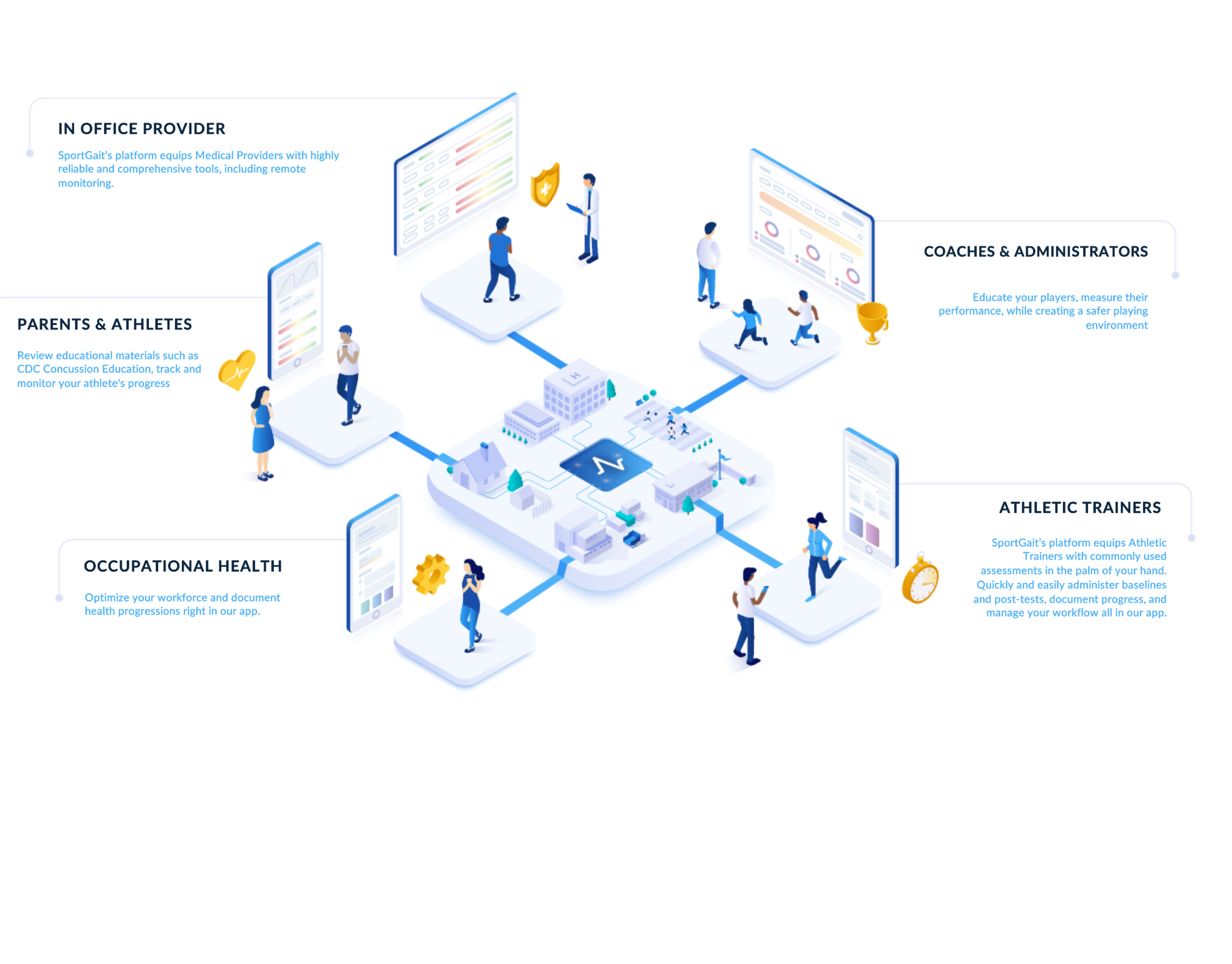
Ecosystem
Accurately measure, identify deficits, optimize performance, and document workflows in our easy-to-use app.
Secure. Private. Compliant.
FDA Listed Medical Devices
CPT Cognitive is currently registered under 21 CFR Part 882. 1470- 510(K) Exempt. Class II. BKG™ Gait is currently registered under 21 CFR 890. 1600 - 510(k) exempt, Class I.
Additionally, SportGait completes regular external audits with AICPA SOC 2 Type II reports, also including legal requirements with HIPAA, HITECH, and applicable state laws.